
Are you a pharma company in need of stability testing but have just too many API or formulation candidates to screen with conventional ICH stabilities on a one-by-one basis? What if we told you there is a new alternative approach which generates accurate stability data three times faster than usual? Whether you are an innovator developing new IMPs without previous stability data or a generic manufacturer in need of minor formulation changes or package screenings, you will be interested to learn more about accelerated predictive stabilities and the comprehensive service we at QUINTA are providing to our clients.
Justyna Hofmanová, PhD in Pediatric Formulation and Josef Coufal, Master in Physical Chemistry, work together at Quinta’s Research and Development department (RAD) as Development Analyst and Project Manager respectively. They both have been involved in multiple projects with a new groundbreaking system: Our accelerated stability assessment services utilize the ASAPprime® stability assessment software licensed from FreeThink Technologies, Inc. Dr. Hofmanová introduces us to this new technology:

From right to left: Justyna Hofmanová and Josef Coufal studying APS data.
“We have numerous clients in Ireland, Belgium and Finland requesting software-assisted accelerated predictive stabilities (APS)” she begins. “APS programs allow us to determine drug substance and drug product shelf life much faster and more accurately than conventional stability testing. To put this in numbers, ICH stabilities require up to a year, while assays under accelerated conditions can still take up to six months for you to decide which formulation is best. But with APS we can screen multiple formulations in just two months: One to stress the samples and another for analysis, design, and performance. That’s three times faster, and we all know time is money especially in pharmaceutical development” Dr Hofmanová claims.
Justyna and Josef agree that time is critical in API, formulation, and product development; and that anything that allows for faster stability testing can have a major impact on the entire process. Nevertheless, both colleagues stress that the advantage of this new approach isn’t just speed but also accuracy. Josef Coufal elaborates further:
“In traditional accelerated stabilities we accelerate aging by exposing samples to high temperatures and humidities and then predict shelf life at normal conditions” Mr. Coufal explains. “The problem is that most solid-state reactions often exhibit non-linear kinetics and simple extrapolation of the kinetics does not accurately predict degradation rates at long-term storage conditions.” He continues: “The accuracy of ASAPprime® hinges on two important ideas: Isoconversion and the extended Arrhenius equation. With isoconversion, samples are stressed under different conditions and the model computes the time (the so called isoconversion time) necessary to reach a fixed degradation limit at each condition. Then the extended Arrhenius exponential equation is used to extrapolate isoconversion times to standard temperatures but also taking into account the humidity factor. Such approach provides more accurate predictions than traditional kinetics extrapolations” Mr. Coufal concludes.
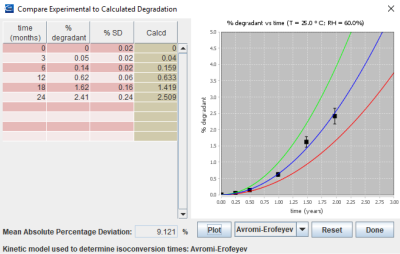
ASAPprime® comparison between calculated and experimental degradation
At this point the two experts bring to the table what makes Quinta-Analytica the best partner to explore the full potential of predictive stability models. Justyna Hofmanová carries on:
“APS programs are very powerful tools, but as such they also require knowledgeable users who will correctly interpret all input and output data in order to trust the accuracy of the modelling and its final predictions: Like in all models, if you put rubbish in, you get rubbish out” Dr. Hofmanová declares. “In this approach it is important to collect data from previous accelerated stability tests, which APS software employs to identify the most suitable stressing conditions for your samples, proposes a statistically representative study design and finally we perform stress stability testing under the conditions recommended by the software. Fortunately, we at Quinta have extensive analytical experience to acquire reliable data and assess the quality of the predictions generated. Without that critical approach, even the finest software will fail to provide correct answers.” As Dr. Hofmanová concludes, Mr. Coufal takes over:
“And provided that we have quality data, there are both innovator and generic cases where stability predictions can be accepted in regulatory filings” he asserts. “We work with generic sponsors in need of minor post-approval modifications, as change of packaging materials or limited, quantitative changes in formulation, which authorities may accept after the provision of predictive comparative studies confirming that changes have no impact on shelf life. Naturally, this doesn’t mean we can consider such software-assisted studies a definite substitute for standard ICH stabilities, but rather a useful tool to speed up non-GMP early development stages” Mr. Coufal clarifies. “Formulation and API developers with tons of different candidates cannot afford to perform full-fledged stabilities on a one-by-one basis but can quickly choose the best ones with software-assisted APS for GMP-stage stability testing” he defends.
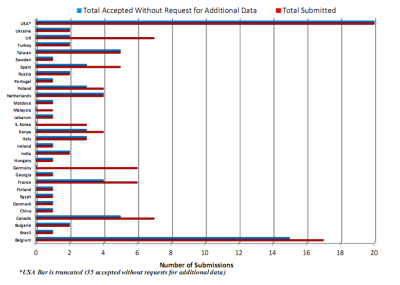
Regulatory experience with Predictive Stabilities by country. Reprinted with permission of FreeThink Technologies, Inc.© 2022
“Speaking of which” Justyna interjects, “while our RAD department focuses on non-GMP analytical testing, the rest of our group manages over a hundred ICH and ongoing GMP stabilities every year, with an entire team dedicated to stability testing, 250m3 of climatic chambers at all conditions required by ICH in addition to photostabilities, freeze-thaw studies, and other non-ICH assays.” She continues: “But it is clear that GMP certifications aside the speed of generating stability data for new drug candidates will remain a key limiting factor. In recent years we have observed a global increase in the number of approved submissions assisted by predictive stability assays. Consequently, in those cases where such predictions are suitable, I believe pharma companies will employ APS tools more and more often, and we will be happy to assist them in their learning process” Dr. Hofmanová finishes.
QUINTA-ANALYTICA is an EMA and FDA-inspected European CRO, part of the Conscio group. For over 25 years Quinta has offered GCP/GLP/GMP-certified clinical, bioanalytical and CMC services for the pharma and biotech sectors. Click the links for more information about Quinta’s stability studies, analytical R&D or contact us at sales@quinta.cz.
Article by Albert Pineda




